PDF Version: CCS Understanding Prototype Development Phases
Background
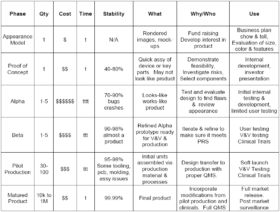
Having a common understanding of the functional utility for each stage of prototype development is essential to managing the expectations for everyone involved in a project, from the designers to the executives and the investors. Prototypes at each development stage have a utility that needs to be understood to justify the cost and time to develop them. Here is a chart that presents the prototype development stages and the effective use of the prototypes at each level.

Appearance Model
The appearance model may be rendered images from an industrial designer or a physical mock-up made from foam board or 3D printing. It may look like the final product. It is used to demonstrate the size, colors, control locations, actuator size/location and other visual features. In some cases it may be a series of drawings that explores a number of configurations for the product. It should be done in weeks rather than months. It may be used to gage investor interest and to get end user feedback. It is part of the concept design of the system.

Proof of Concept
Proof of concept (PoC) prototypes are bench top physical mock-ups and breadboards. They are used to evaluate the performance of a subsystem or technical component for feasibility. For example, a tubing clamp design could be evaluated for pressure withstand or flow control capability or a dc-dc converter could be evaluated for heating under load, noise, and regulation. PoC prototypes may be used to evaluate the usability of a user interface, such as the operability of an active graphical user interface (GUI) mock-up or the ease of tubing kit loading onto a pump panel mock-up. These evaluations and feasibility reports assist with component selection and specification development and are part of the design history file for a medical device. The PoC prototypes designs are 40% to 80% stable for the final design. The PoC prototype stage should be done in a few months.
Alpha
The Alpha prototype is the initial attempt at designing and fabricating the product to meet the Product Requirements Specification (PRS). It is also the first attempt at making it look like the final product and work like the final product. The iterative process of designing and building the Alpha prototype will provide the guidance for the next stage. The Alpha may be constructed with 3D printed enclosures and components for physical fit and performance evaluation. It will have initial PCB and enclosure designs for internal testing and evaluation of performance, safety, EMC, usability and appearance. The Alpha development is expensive compared to previous stages, and requires months to iterate and refine the design. The Alpha design and testing is essential in understanding the limitations of the product and in refining the design.

Beta
The Beta prototype development incorporates the design refinements found in Alpha development and implements them into production tooling, molds, PCBs, subassemblies, enclosures, GUI designs, etc. Test plans and verification protocols are prepared. Software is refined and prepared for the first release. Documentation is updated and prepared for releasing the device master record (DMR). Production testing and assembly protocols are drafted. The Beta prototypes are assembled and tested per the production procedures. The Beta prototypes are ready for verification and preliminary validation testing, safety and EMC testing, and performance testing to verify compliance with the PRS. There will be refinements that are required after assembly of the Beta prototypes and these refinements should be under configuration control to reflect the reasons for the changes and how they make the Beta prototype overcome any deficiencies in meeting specifications and standards.
Pilot Production
The pilot production phase is where the refinements from the Beta prototype verification and validation testing are incorporated into the design and into the production process. The design transfer to manufacturing and the implementation of the quality management system is done for pilot production. These units may be used for summative usability testing and clinical trials. They are suitable for initial release to market. The design and the production process are relatively stable.
Matured Product
The matured product incorporates the refinements from user feedback and production monitoring. The design and the assembly process are stable, have high yields and incorporate cost saving measures.
Have more questions about the prototyping process? Contact Us
Twitter: @Cooper_Engineer
LinkedIn: Cooper Consulting Service